Offering:
- Exceptional customer focus
- Industry expertise and project management skills
- Competence in matching customer expectations through the life cycle of the project
- Cost-competitive and timely delivery
- Adherence to client process requirements and specifications
- State-of-the-art design and manufacturing technology and equipment
- Eco-friendly value engineering
- Space saving and easy to maintain designs
- Designs in strict compliance with ASME, ASME BPE and GAMP5 specifications
- Cost-competitiveness
- Quality standardization and documentation
- Timely Delivery
Products - Open Tabs for More Information

- Sterile Solutions
- Vial
- Ampoules
- I.V. Fluids
- Aerosols
- LVP & SVP Solutions
- Vaccines
- Injectables
- Human Insulin
- Serums
- Plasma Fractions
- Bacteria and cell cultures
- LVP
- Aseptic Design of bottom outlet valve with zero hold up
- Customised design incorporated for diverse applications
- Calibrated full view glass for Sterile Mixing Vessel
- CIP and SIP style design of vessel, fittings and piping
- Injection ports, injection needles available on the top of the lid
- Volume monitoring by load cells
- Process Automation
- Sterility compliant
- Magnetic drive, so no seals, no shaft, thereby eliminating contamination
- Mixing head designed for vigorous mixing or low volume blending
- Minimum space requirement
- Optimized Bioreactor / Fermentor design
- Range : 5L – 25000L (Customized to user requirements)
- Skid – mounted structure
- Agitator (Top / Bottom mounted)
- Design compliance to ASME BPE and GAMP 5 guidelines
- Material of construction
- Parts in contact with the media : SS 316 L
- Internal surface RA < = 0.4 , Electro –polished
- Single / double mechanical seal
- Easy access during operation and routine maintenance
- Safety features to protect the batch in case of any component failure
- Metered dose inhaler (MDIs)
- Nebulizers
- sublingual’s
- skin sprays (coolants, anesthetics, etc.)
- Dental spray & other similar pharmaceutical aerosols & HFA type products.
- Vessel design as per ASME SEC-VIII, DIV-I
- High Pressure vessel with Agitator.
- Mechanical seal (Garlock) for stirrer to withstand high pressure.
- Zero dead legs valve for outlet.
- Rapture disc for pressure safety.
- Minimum batch stirring agitator with high pressure.
- Vessel is CIP / SIP able.
- Automation in compliance with 21 CFR Part 11
- All Documentation and Validation provided
- Ointment
- Sterile Ointment
- Cream
- Gel
- Lotion
- Shampoo
- Toothpaste
- Other Semi Solid Emulsions
- Complete untouched -Processing Plant
- CIP / SIP able
- All wetted parts are of SS 316
- All Pipelines are seamless & internally Electro-Polished
- Re-Circulation after Mixing (provided)
- Mixing and Dispersing quality adjustable
- Jacketed vessels for heating / cooling processes
- Compliance of cGAMP Guidelines
- The Multi-Mix in Pressure and Vacuum Tight Mixing Vessel.
- All Material Transfer Done Under Vacuum.
- Load cell for accurate weighing
- PLC based control panel / SCADA for process automation
- FLP System can be provided
- Orals
- Liquids
- Suspensions
- Cough Syrups
- Drop Solutions
- Expectorants
- Antacid Suspensions
- Sugar and Glucose Transfer System
- Sugar Syrup Preparation with efficient bottom entry agitation
- Clear and Suspended Liquid Manufacturing System with On-line or Built-in Homogenizer
- Efficient Filtration System for the clear / suspension syrups
- CIP & SIP System for cleaning & sanitization of the system
- PLC based Automation System as per 21 CFR Part 11 (Optional)
- System Qualification Documents like DQ, FAT, IQ & OQ Protocols
- “CE” marked Equipments are offered with Third Party Inspection & All Electrical Control with ERTL Approval & Test
- Food Grade Gaskets in compliance with FDA 21 CFR Part 177-2600
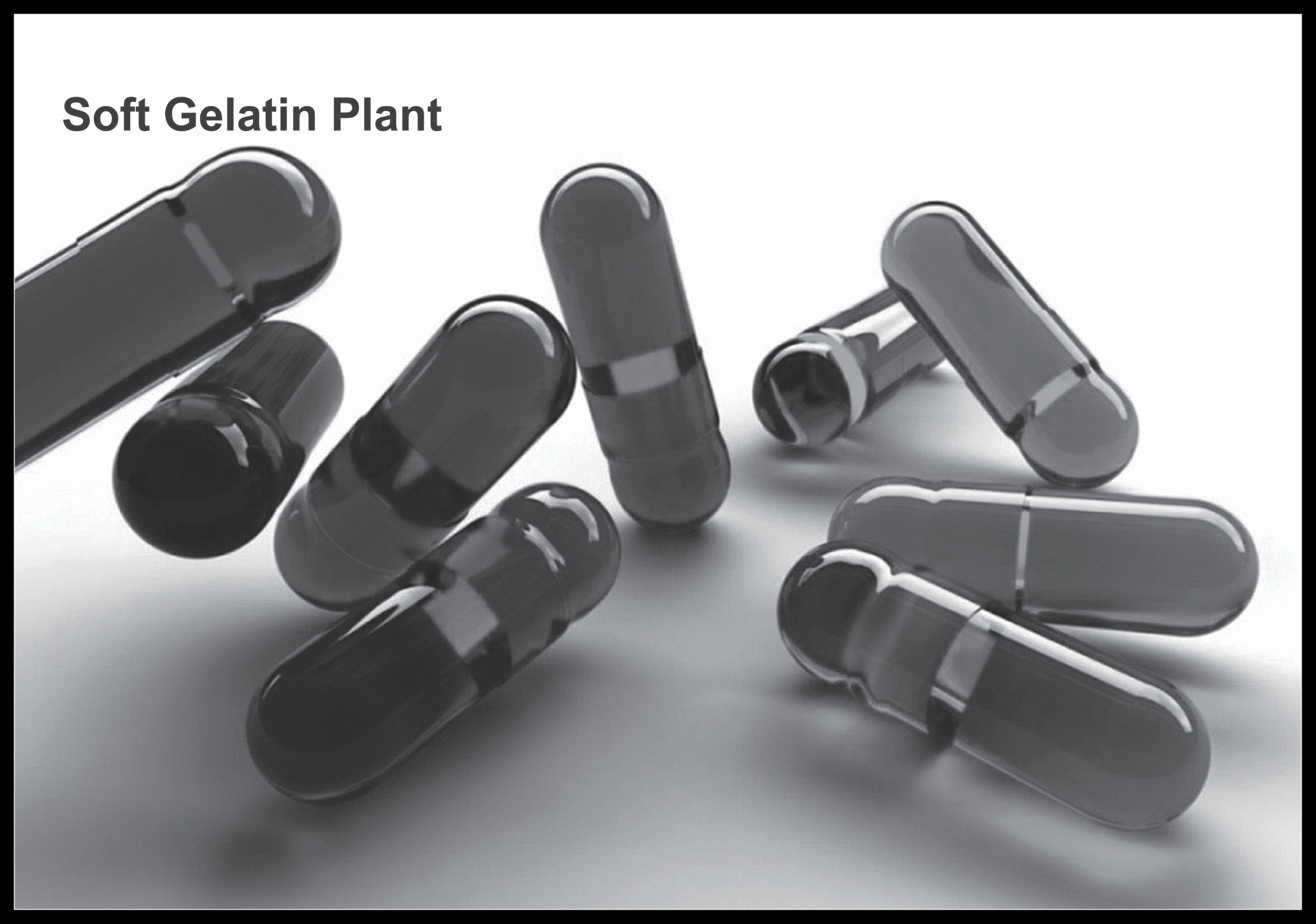
- PLC Programmed (optional) for dual speed agitation to reaction mass of Gel Cooker as per defined recipe.
- Hydraulic Lifting System for top cover of Gelatin Cooker.
- Medicament Mixing & Homogenizing Vessel.
- Multi-purpose Mixing & Deaeration Unit with SS Control Panel for carrying out following operation:
» Mixing of Medicament
» Colour Mixing in Gelatin mass - Deaeration of Gel mass.
- Heat traced transfer pipe line for feeding Gelatin to Encapsulating Machine
- Vacuum Control System for Deaeration in Gelatin Cooker.
- Strict compliance to cGMP as per the International Standard norms.
- SS 316 L contact parts Mirror polished to less than 0.5 Micron RA & SS 304 Non-Contact parts Matt finished.
- 100% drainable, crevice and dead leg free internal surface.
- Specially constructed Gelatin Feed Tank with Electronic Heating System.
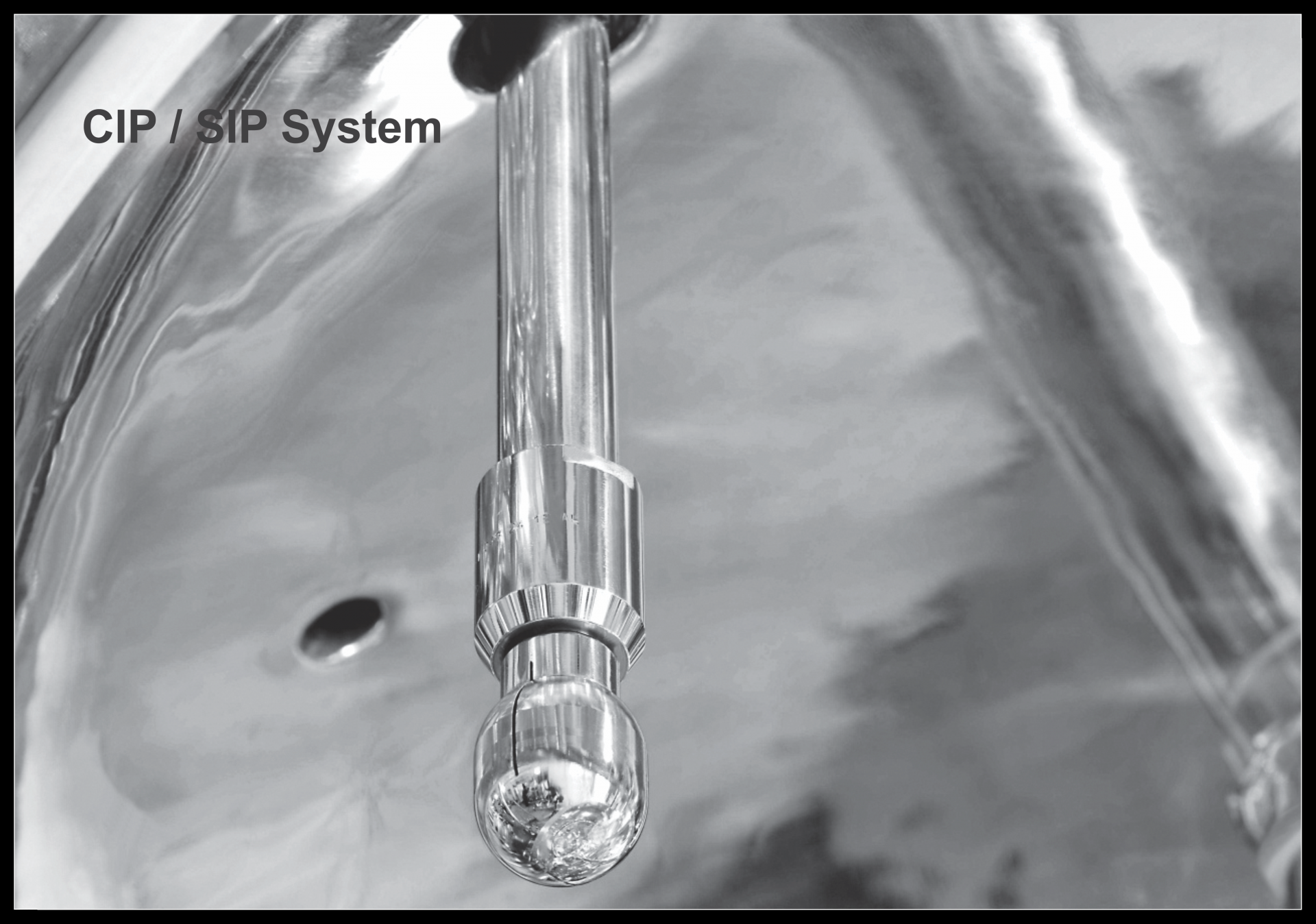
- CIP Tank/Tanks, Jacketed/Non-Jacketed
- Supply Pump/Return Pump
- Heater as required
- Dosing Tanks/Pumps
- Inter-connected Pipings / Valves / Instrumentation
- Automation System/Control Panel
- SS Skid/SS Platform
- Pure Steam Header with PI, TI & Safety Valve
- Condensate Header with sterile steam trap unit and Temp. Sensor
- PLC Control Panel with Time / Temperature control program, printer, alarm etc. as required
- SS Mobile Stand with PU Castors
- Sanitary Design to meet all cGMP criteria.
- Contact parts : SS 316 L, Mirror polished & EP to < 0.5 Micron finish
- All Non- Contact parts : SS 304 Matt finish
- Orbital Tube welding
- Automation as per MCA & USFDA 21 CFR Part 11
- Built to custom requirement
- 100% Drianability
- GMP documentation like DQ, FAT, IQ, OQ & PQ Protocols
- Site Installation & Commissioning
- Documented Output of cleaning & sterilization cycles

- Cheese
- Chocolate
- Ice cream
- Tomato ketchup
- Mayonnaise
- Tea
- Lozenges
- Hair dye
- Inhaler
- Disinfectant
- Agro Chemicals
- Food and Beverages
- Breweries and Distilleries
- Oils, Paints and Resins
- Consumer Goods
- Cosmetics
